
Virtual Back to School Night
Grade 6 Science and Outdoor PE Video
Grade 7 Science and Outdoor PE Video
Grade 8 Science and Outdoor PE Video

Grade 6 Science and Outdoor PE Video
Grade 7 Science and Outdoor PE Video
Grade 8 Science and Outdoor PE Video


Grade 6 into 7 Prep. Links and Resources
ECOSYSTEMS
Biotic vs. Abiotic Factors
Build your own Self Contained Biosphere student video
Food Webs
Ecosystems and Biomes link and video
Ecosystems Terminology and Organization
CHEMISTRY
Lab Rubric
Matter and the Particle Theory
The Periodic Table of Elements
Some Key Concepts About Elements and their Atoms
HEAT
What is Heat?
Thermal Expansion Video
Heat Transfer – Conduction, Convection, and Radiation
Radiant Energy
States of Matter and the Changes of State
STRUCTURES
Structure Types
Grade 7 into 8 Prep Links and Resources
CELLS
Plant Cell Organelles
Cell Structures
Compound Microscope Parts
Preparing Slides
Diffusion and Osmosis
FLUIDS
Properties of Fluids: Viscosity
Properties of Fluids: Density
What is Density and How to Calculate Student Note
Density Explained
Calculating Density and Buoyancy Online Simulator
Archimedes Gold Crown Problem – Solved using Density
Fluids Under Pressure: Hydraulics vs. Pneumatics video
SYSTEMS IN ACTION
6 Types of Simple Machines
Net Forces Explanation
Levers – Class 1, Class 2, Class 3
Calculating Mechanical Advantage
Gears
Pulleys and Mechanical Advantage
ELECTRICITY
What is Static Electricity?
Components of Electric Circuit
How Solar Energy is Transformed into Electricity
Series vs Parallel Circuits
Build Your Own Circuit Simulator
Grade 8 into 9 Prep Links and Resources
CHEMISTRY
Properties of Matter (Physical properties vs Chemical properties)
Changes in Matter (Physical changes vs. Chemical changes)
Elements and Reading the Periodic Table
Interactive Periodic Table of Elements
Some Key Concepts About Elements and their Atoms
How to Draw Bohr Rutherford Diagrams
Covalent vs. Ionic bonding
BIOLOGY
Food Chains and Food Webs
Biogeochemical Cycles
BIODIVERSITY
ELECTRICITY
Static Electricity
Charging and Object by Induction
Series and Parallel Circuits
SPACE
The Milky Way
Outer Space
The Solar System
The Moon
Moon Phases
Comets, Asteroids, Meteors, Meteoroids, Meteorites

All presentations, links, and videos from the Structures: Form and Function lessons will be posted below.
Stability and Forces Presentation
Stress on Structures and Structure Types Presentation
Stability, Symmetry & Bridge Type Presentation

Find all presentations, videos, screencasts, handouts, and assignments below!
MUST DO
Lesson 1: Introduction to Heat
PDF of Intro to Heat Slides Presentation
Estimating Temperatures Handout
Lesson 2 : Thermometers PDF (3 HW questions located on last slide)
Lesson 3: Heat, Temperature and the Particle Theory
Thermal Expansion Video
PDF copy of presentation
Bill Nye: Heat worksheet link (make a copy and share with Mr. Ray)
Lesson 4: Conduction
PDF of presentation
Lesson 5: Convection
Lesson 6: Radiation
Lesson 7: States of Matter
SHOULD DO
Learn how to code, tell stories, and make games using Scratch
Friday June 12 Lesson: Imagine A World in Scratch Coding Document (link)
COULD DO
Ear Saver .stl file for use in TinkerCAD or Blender
Practice your coding skills with code.org Minecraft or Dance party activities

Below are the resources we went over in class when learning about the periodic table. Play around with the simulator at the bottom of the post if you want to learn more!
Some Key Concepts About Elements and their Atoms
How to Read a Box on the Periodic Table of Elements (google doc link)
Build an Atom Simulator student assignment (Due Wed. May 6th)

Here is the presentation we went over together on the Particle Theory. You can refer back to it when completing future at home labs.
Visit the following website (https://www.proprofs.com/quiz-school/story.php?title=particle-theory) and complete the quick quiz on the Particle Theory.
Dissolving Rates
Here are the links to the at home labs for this unit
Marks are based on the Scientific Method in this Lab Rubric
Lab # 1 – Matter – Due March 31, 2020
Lab # 2 – Dissolving Rates – Due April 3, 2020

Please watch the two videos below and then answer the questions at the bottom of the post in a google doc. Please share your doc with me j.ray@theojcs.ca and have your answers with you when we meet during Monday’s live session. I hope everyone is doing well, and there is no baby update except that our bags are packed and ready at any point!
Before you answer the following questions, please create a folder in your google drive called ‘Science’ to put all of your docs/resources for Science in one spot and keep you organized.
Questions – submit #1-4 to me (Challenge question is an extra and not mandatory)
a) wood b) tap water c) orange juice d) loonie coin
4. Give an example of each of the following kinds of solutions, other than those mentioned in the video.
a) a liquid in a liquid b) a solid in a solid c) a solid in a liquid
Challenge Question
Sea water contains perhaps the largest number of dissolved solids. Gold, for example, is found dissolved in sea water. It has been estimated that if 1 km³ of sea water is evaporated, 0.004 tonnes of gold can be recovered. What is the value of the gold in 1 km³ of sea water? (You can check online to see what the current price of gold is. Gold is often measure in troy ounces, and one troy ounce = 31.1 g)
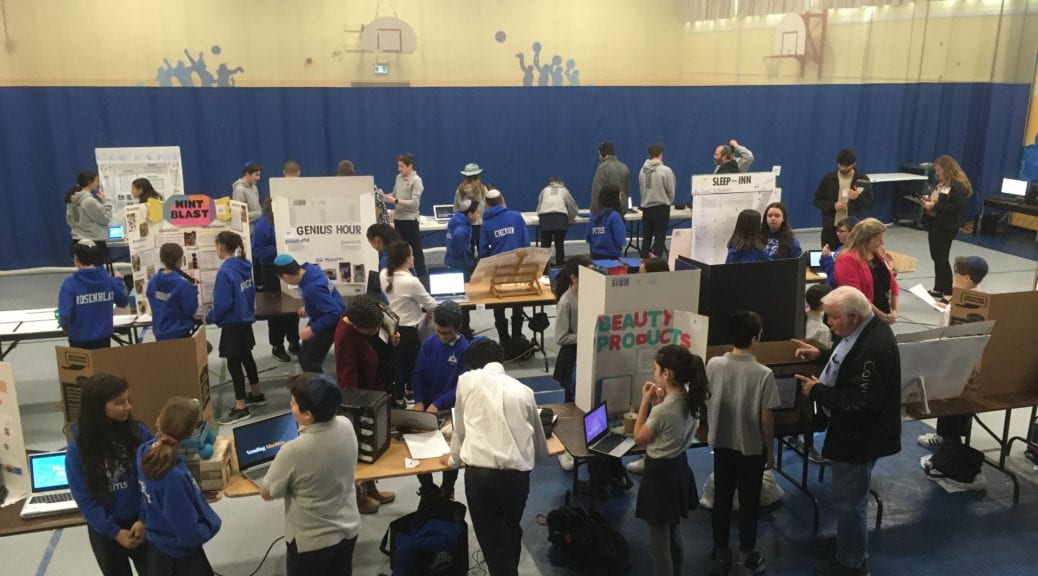
Congratulations to the middle school students for successfully accomplishing their projects this year! I heard so many positive and amazing comments from parents, teachers, and this year’s judges. They were impressed with the level of research, the creative engineering, and imaginative presentations that YOU designed and created. Outstanding effort, I am very proud of the work you put into those projects! Here are the results from Innovation Day judging as well as the video we saw as part of the assembly. I can only imagine what creative projects you will dream up for next year. Well done!
Genius Hour Project Winners
Grade 6
1. Samara S. – indestructible dog toy
2. Maya S. – Magical illusions
3. Rebecca G. – Nail glue
Grade 7
1. Maayan S. – The perfect environment for horses
2. Sarah K. – A dollar makes a difference
3. Yamaya N. – Eco-friendly housing
Grade 8
1. Jayson R. – Braille Rubik’s cube
2. Zoe N. – Eco-friendly dill
3. Max P. – Music Production
STEAM Project Winners
Grade 8
1. Talia C. and Jesse A. – Clean Machine
Grade 7
1. Abby T. – Music and Memory
2. Sarah N. and Jordana W. – Mint Blast
3. Talia L. and Sasha S. – Sleep-Inn

Innovation Day is finally upon us! It feels like forever ago when students began thinking about their passions, ideating different topics, creating pitches, and starting the journey of their personalized learning. As the teacher, I can’t tell you how excited I am to see all of the hard work put into researching their topics, the creative problem solving needed to engineer products, and the creative story they will tell, all come together for next week’s presentations.
Here is a schedule for both students and parents to look over to see how the set up, parent visitation, judging, and final awards ceremony is scheduled.
Wednesday, March 4th
2:25 – 3:05 Set up for grade 6, 7, and 8 (gym)
3:05 – 4:00 Open to parents to visit grades 6 to 8 projects (gym)
Thursday, March 5th
9:00 – 10:50 Judging of Grade 6, 7 and 8 STEAM and Genius Hour Projects (by external judges)
8:45 – 9:30 Open to parents and classes to visit grades K to 5 Projects in their classes.
10:50 – 11:10 Tefillah for grade 6 to 8 students.
11:10 – 11:25 Snack break in regularly scheduled class with teacher for grade 6 to 8 classes followed by class as usual
11:05 – 1:05 Grade 6 to 8 projects left up in the gym for K – 5 classes to visit with their teacher
1:45 – 2:25 Clean-up of grades 6 to 8 Projects in gym
3:15 Whole School assembly to announce the grade 7 and 8 bronze, silver, and gold medalists